4s23d6|electronic configuration in periodic table : Pilipinas The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements .
METRO Fast bet. 746 likes. Nonprofit organization
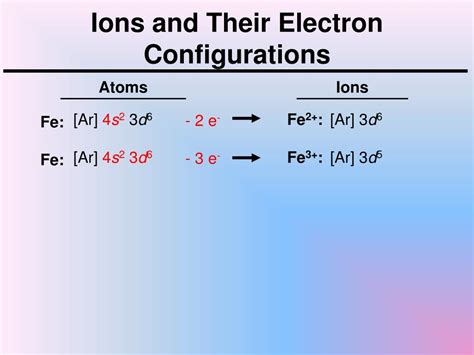
4s23d6,Which element has the following electron configuration Ar 4s23d6? Updated: 8/11/2023. Wiki User. ∙ 11y ago. Best Answer. Iron has the electron configuration [Ar]3d64s2. Wiki User.
Iron (atomic symbol: Fe; condensed electron configuration: [Ar]4s23d6 and an electron box configuration as seen in the figure above) exhibits typical metallic behavior. Bulk iron has . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at .
The Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elements!From Sc on, the 3d orbitals are actually lower in . Re: electronic configuration in periodic table. You can look at the orbitals in two useful ways. The energy of the orbitals and the location of the orbitals. When .

The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements .
The Order of Filling Orbitals. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. Where . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic .

Video: Fe, Fe2+, and Fe3+ Electron Configuration Notation. In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only .Video: Fe, Fe2+, and Fe3+ Electron Configuration Notation. In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only . Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.The chemical symbol for Iron is Fe. . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron .Video: Fe, Fe2+, and Fe3+ Electron Configuration Notation. In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.We would like to show you a description here but the site won’t allow us.
But, the orbitals overlap. The Madelung rule gives the order: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p. Oganesson (element 118) is a good example to show the order of the orbitals. Its electron configuration is: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s . Place the following elements in order of decreasing atomic size (Cl, P, S) n=4 to n=2n=3 to n=2. Select the transitions that emit visible light in the hydrogen atom. Think about the Bohr model of the atom while answering this question.n=7 to n=8n=2 to n=6n=4 to n=2n=3 to n=6n=3 to n=2. Number of Core Electrons: 46Number of Valence .4s23d6 electronic configuration in periodic table An element with 4s23d6 configuration loses 2 electrons from the 4s subshell. An element with 2s22p5 configuration gains 1 electron into the 2p to form a -1 ion. Explanation: If an element with the valence configuration 4s2 3d6 loses 2 electrons, these electrons would be prominently removed from the 4s subshell. TA获得超过1.6万个赞. 关注. 是这样的,在多电子原子中,由于屏蔽效应和钻穿效应,导致 原子轨道 的能级发生交错,致使3d轨道的能量高于4s轨道,必须先填满4s轨道才能填充3d轨道. 所以铁的电子组态是1s2 2s2 2p6 3s2 3p6 4s2 3d6. 更多追问追答 . .
Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..An element 'M' has atomic number 12. (a) Write its electronic configuration. (b) State the group to which 'M' belongs. (c) Is 'M' a metal or a non-metal.
The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 4.4.2 4.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr. The 4s23d6 electrons. Iron thus has 8 valence electrons! Easy-Peasy, once you know the trick! Note: Just because iron has 8 valence electrons doesn't mean that it will use them all. Iron usually . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and . Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom. The electron configuration of a oxygen atom is. O: 1s22s22p4 (1.9B.1) (1.9B.1) O: 1 s 2 2 s 2 2 p 4. which may be shorted.
The Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elements!From Sc on, the 3d orbitals are actually lower in .4s23d6 Điện phân dung dịch hỗn hợp chứa 0,04 mol AgNO3 và 0,05 mol Cu (NO3)2, điện cực trơ, dòng điện 5A, trong 32 phút 10 giây. Khối lượng kim loại bám vào catot là: Cho x mol Fe tác dụng với y mol AgNO3 đến phản ứng hoàn toàn thu được dung dịch chứa hai muối của cùng một kim loại .
electronic configuration in periodic table Điện phân dung dịch hỗn hợp chứa 0,04 mol AgNO3 và 0,05 mol Cu (NO3)2, điện cực trơ, dòng điện 5A, trong 32 phút 10 giây. Khối lượng kim loại bám vào catot là: Cho x mol Fe tác dụng với y mol AgNO3 đến phản ứng hoàn toàn thu được dung dịch chứa hai muối của cùng một kim loại .
How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are simple. When we write the configuration we'll put all 24 electrons in orbitals .
Electronic Configuration: The ground-state electronic configuration of an element is the distribution pattern of electrons into the shells and subshells. Iron has the abbrieviated elcetron configuration of [Ar] 4s23d6. What is Ne 3s2 and Ne 3s2 and Ar 3d6 4s2 noble gas notation for? Ne 3s2 would be Magnesium, and Ar 3d6 4s2 is Iron.
4s23d6|electronic configuration in periodic table
PH0 · electronic configuration in periodic table
PH1 · Which element has the following electron configuration Ar 4s23d6
PH2 · The Order of Filling 3d and 4s Orbitals
PH3 · Iron
PH4 · Electron configurations of the 3d transition metals
PH5 · Electron Configuration of Transition Metals
PH6 · Electron Configuration for Iron (Fe, Fe2+, and Fe3+)
PH7 · Electron Configuration Calculator
PH8 · 5.17: Electron Configurations and the Periodic Table